23/11/2025 - Check out our review on the
solubility, conformation and supramolecular aggregation of carboxymethyl cellulose
.
08/11/2025 - We have a new pre-print on the
solution behaviour of pullulan in aqueous media.
05/11/2025 - Optilab rEX differential refractometer arrives to our lab
15/10/2025 - New freeze dryer from Stony lab arrives to our lab
06/09/2025 -
Pre-print on the behaviour of polyelectrolytes in organic solvents. Special thanks to the SPring-8 synchrotron and the ILL for all the beamtime they gave us to complete this work.
27/07/2025 - Check out
our latest preprint on ion-pairing and counterion condensation in carboxymethyl cellulose solutions.
27/06/2025 - Our latest work on the influence of counterion type on polyelectrolyte phase bahaviour
is out on Macromolecules
20/06/2025 -
New paper on the interaction of nanoions with cellulose ethers published in JCIS - Chaotropic or Hydrophobic Effect: Distinct binding signatures of nano-ions to a non-ionic polymer - in collaboration with
Max Hohenschutz from RWTH Aachen.
17/02/2025 -
New paper published in Nanoletters - Nanoparticle Loading in Swollen Polymer Gels: An Unexpected Thermodynamic Twist - in collaboration with
Rob Hickey .
04/02/2025 -
BI-DNDC refractometer from Brookhaven arrives to our lab.
09/01/2025 - Density and speed of sound meter
DSA 5000 arrives to our lab.
13/12/2024 -
Preprint on the interaction of nanoions with polysacchrides is out.
22/10/2024 -
Paper on the influence of counterion type on the scattering properties of polyelectrolyte is published in
Soft Matter. All the data for this study are
available .
19/09/2024 - Our
Paper on polyelectrolytes in mixed solvents published in
Carbohydrate Polymers, this is a collaboration with prof.
Takaichi Watanabe from Okayama University. All data are availble
here.
07/08/2024 - Check out our latest
preprint on the influence of counterion type on the scattering properties of semiflexible polyelectrolyte carboxymethyl cellulose.
05/08/2024 - Our
letter on the viscosity of polystyrene sulfonate with different counterions in aquoues and organic solvents is out in
ACS Macro Letters .
20/07/2024 - Our
paper on the rheology of polystyrene sulfonate in aqueous salt solutions is published in Macromolecules.
01/05/2024 - Elmira Gharehtapeh joins the group
11/04/2024 - Lingzi Meng joins the group
18/01/2024 - Read our latest
preprint on the influence of counterion type and solvent on the viscosity of polyelectrolytes solutions.
18/01/2024 - New article on mechanically robust polymerised ionic liquid Networks is
published.
01/01/2024 - Group officially starts at Penn State
14/12/2023 - Read our
latest pre-print on the properties of a cellulose-based polyelectrolyte in mixed solvent media. Special thanks go to the
Spring-8 synchrotron for awarding us beamtime to do the SAXS experiments.
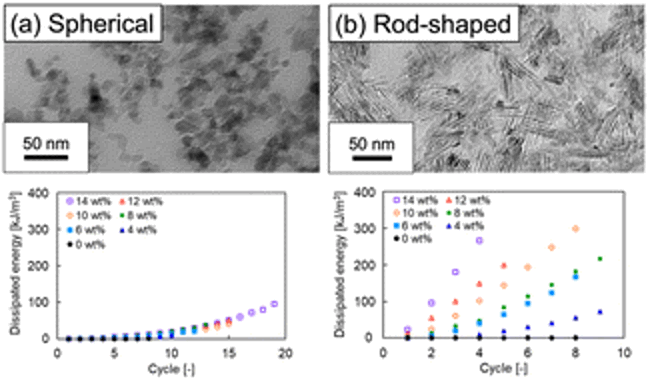
07/12/2023 - Read our
latest work on the re-inforcement of of polymerised ionic liquid gels with anisotropic particles .
01/12/2023 - Read our latest
pre-print on the solution properties of polymerized ionic liquids. This work is a collaboration with the groups of prof.
Takaichi Watanabe from Okayama University, prof.
Atsushi Matsumoto from the University of Fukui and prof.
Walter Richtering from RWTH Aachen in Germany. Special thanks go to the ISIS and J-PARC neutron sources and the Diamond Light Source and Spring-8 synchrotrons for the beamtime awarded.
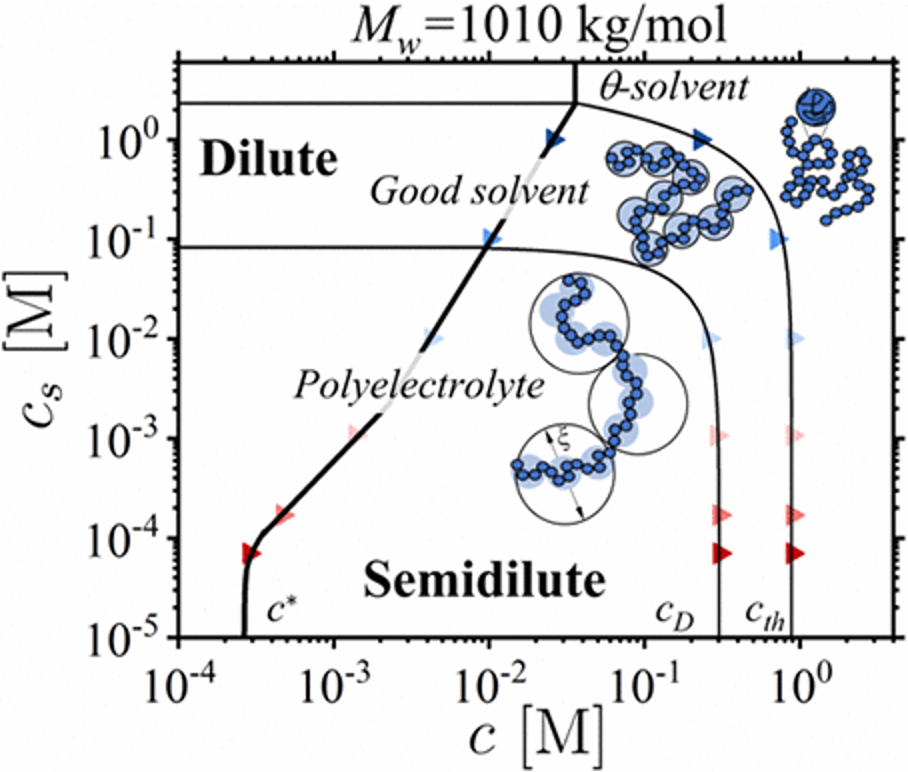
22/11/2023 - Review on polyelectrolytes in dilute solution is accepted into
Soft Matter. This paper was written in collaboration with prof.
Atsushi Matsumoto from the University of Fukui and
Amy Shen from OIST. You can find a pre-print
here.
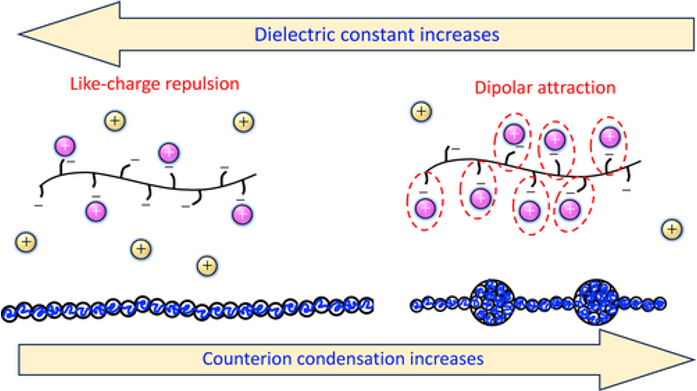 Electrostatically-Driven Collapse of Polyelectrolytes: The Role of the Solvent's Dielectric Constant
(Journal of Polymer Science, 2025)
Electrostatically-Driven Collapse of Polyelectrolytes: The Role of the Solvent's Dielectric Constant
(Journal of Polymer Science, 2025)