Welcome to our group website. We study the structure and dynamics of polymers in solutions and in networks using small angle scattering and rheological techniques. We focus on ion-containing polymers, especially polyelectrolytes and ionomers.
 Electrostatically-Driven Collapse of Polyelectrolytes: The Role of the Solvent's Dielectric Constant
(Journal of Polymer Science, 2025)
Electrostatically-Driven Collapse of Polyelectrolytes: The Role of the Solvent's Dielectric Constant
(Journal of Polymer Science, 2025)
Anish Gulati, Lingzi Meng, Takaichi Watanabe and Carlos G. Lopez
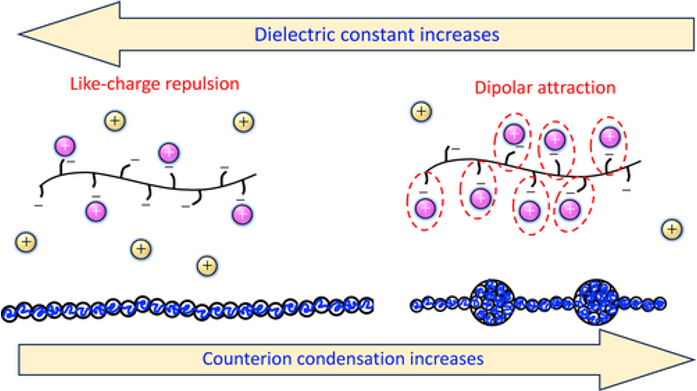
PolyelectrolytesWe experimentally confirm a longstanding theoretical prediction of counterion-induced polyelectrolyte collapse in low dielectric media. The scattering behaviour of polystyrene sulfonate in different solvents with dielectric permittivities in the range of \(\epsilon \simeq\)12−180 is investigated. For high and intermediate ε media, typical polyelectrolyte behaviour is observed: the correlation length (\(\xi\)) scales with concentration (c) as \(\xi \sim c^{−1/2}\), as predicted by various theories. When the dielectric constant of the solvent decreases below \(\simeq\) 22,
a scaling of \(ξ ∼ c^{−1/3}\), characteristic of partially collapsed polyelectrolytes, is observed. Chain collapse is further supported by the disappearance of the correlation peak at high polymer concentrations. Interestingly, polyelectrolyte collapse is observed under both solvophilic and solvophobic conditions, supporting the existence of attractive electrostatic interactions. These results are in qualitative agreement with theoretical predictions which expect chain collapse in low dielectric media due to the influence of condensed counterions, either via dipolar attraction and/or charge-correlation-induced attractions.
All data for this study can be downloaded here.
 Solutions of Carboxymethylcellulose with Organic Counterions (I): The Influence of Counterion Properties on the Polymer Structure and Solubility (Macromolecules, 2025)
Solutions of Carboxymethylcellulose with Organic Counterions (I): The Influence of Counterion Properties on the Polymer Structure and Solubility (Macromolecules, 2025)
Can Hou, Walter Richtering, Takaichi Watanabe, Kai Leonhard, Maxim Papusha and Carlos G. Lopez
Polyelectrolytes often display good solubility in water but not in organic solvents, a feature that limits their applications in nonaqueous media such as hand sanitizers. Here, we show that this limitation can be overcome by tuning the counterion–solvent affinity. To this end, the solubility and chain conformation of carboxymethylcellulose (CMC) salts with different organic counterions in a variety of solvents were studied by employing the Hansen solubility parameter (HSP) framework and small-angle X-ray scattering (SAXS), respectively. The solubility phase mapping demonstrates an increase in the soluble region in HSP space for the polyelectrolyte to encompass more solvents as the counterion side arm length increases or if the side arm is substituted with a large functional group, while substituting the central atom does not change the solubility, suggesting that the solubility is mainly influenced by the interaction between the peripheral atoms and the solvents. Scattering measurements revealed that for a given solvent, the nature of the counterion does not influence the conformation of chains in solution, as seen by the independence of the stretching parameter B on counterion type.
All data for this study can be downloaded here.
 Structure and rheology of carboxymethylcellulose in polar solvent mixtures (Carbohydrate Polymers, 2025)
Structure and rheology of carboxymethylcellulose in polar solvent mixtures (Carbohydrate Polymers, 2025)
Can Hou, Takaichi Watanabe, Carlos G. Lopez and Walter Richtering
We study the conformational, conductometric and rheological properties of semiflexible polyelectrolyte carboxymethyl cellulose in mixtures of water and three non-solvents (ethanol, isopropanol and acetone). Small angle x-ray scattering measurements of the correlation length reveal that the local conformation of the carboxymethyl chain is unchanged by the presence of a non-solvent, even for solutions not far from the solubility boundary. Rheological measurements confirm the invariance of the correlation length upon non-solvent addition. Conductivity measurements show that as the non-solvent content is increased, the fraction of condensed counterions increases, presumably due to the lowering of the dielectric constant of the solvent media. These results therefore show that under room temperature and pressure, the conformation of polyelectrolyte chains is independent of the effective charge fraction of the backbone. We suggest this occurs because the bare Kuhn length (10 nm) is much larger than electrostatic blob size, which is itself of the order of the Bjerrum length (0.7 − 2 nm).
All data for this study can be downloaded using this link.
 Influence of counterion type on the scattering of a semiflexible polyelectrolyte (Soft Matter, 2024)
Influence of counterion type on the scattering of a semiflexible polyelectrolyte (Soft Matter, 2024)
Anish Gulati, Jack Douglas, Olga Matsarskaia and Carlos G. Lopez
Understanding how counterion and backbone solvation affect the conformational and thermodynamic properties of polyelectrolytes remains a key challenge. We address this by studying semidilute aqueous solutions of carboxymethyl cellulose (CMC) with alkaline and tetra-alkyl-ammonium (TAA) counterions using small-angle neutron and X-ray scattering (SANS and SAXS). These techniques probe concentration fluctuations of the polymer backbone and counterions. In SAXS, contrast arises mainly from the polymer backbone for both types of CMC salts. In SANS, however, the contrast is dominated by counterions for TAA salts and by the backbone for alkaline salts. The scattering function exhibits a correlation peak independent of counterion type at low concentrations. At concentrations above 0.1 M, peak positions from SANS and SAXS for CMC with TAA counterions diverge, suggesting a decoupling of fluctuation length scales between counterions and the polymer. An upturn in low-q scattering intensity indicates long-range compositional inhomogeneities, whose strength decreases with increasing counterion–solvent interaction strength (measured by the viscosity B coefficient) and is strongest for the sodium salt of CMC.
All data for this study can be downloaded using this link.
 Viscosity of Polyelectrolytes: Influence of Counterion and Solvent Type (ACS Macro Letters, 2024)
Viscosity of Polyelectrolytes: Influence of Counterion and Solvent Type (ACS Macro Letters, 2024)
Anish Gulati and Carlos G. Lopez
We study the viscosity of polystyrenesulfonate with sodium and tetrabutylammonium counterions in aqueous and organic solvent media. We find that at low concentrations the Fuoss law (\(\eta_{sp} \sim c^{1/2}\)) is approximately obeyed, but at higher concentrations, an exponential dependence on the polymer volume fraction sets in. These findings are discussed in terms of Fujita’s free volume theory.
'.
All data for this study are available as supporting information.
 Rheological Properties of Concentrated Sodium Polystyrenesulfonate in Aqueous Salt Solutions (Macromolecules, 2024)
Rheological Properties of Concentrated Sodium Polystyrenesulfonate in Aqueous Salt Solutions (Macromolecules, 2024)
Anish Gulati, Aijie Han, Ralph H. Colby and Carlos G. Lopez
The traditional view of polyelectrolytes in concentrated salt solutions is that they behave as neutral polymers in good solvent. This is an apt description when the polyelectrolyte concentration is low, but fails at high polymr concentrations. In this paper, we study the conformation and rheology of sodium polystyrenesulfonate in aqueous NaCl solutions in the high polymer, high added-salt concentration regime. For low added salt concentrations, the specific viscosity decreases with added salt as expected. At very high salt, the specific viscosity is found to rapidly increase with increasing added-salt concentration (\(c_s\)). This indicates that addition of salt modifies the system in ways other than simply decreasing the electrostatic screening length. Beyond a critical shear stress of around 400 Pa solutions display strong shear thickening reminiscent of shear-induced gelation. Scaling laws for the zero shear rate viscosity and critical shear rate with molar mass, polymer and added-salt concentration are established and compared to similar behavior observed for other systems. SANS experiments using the zero-average-contrast technique reveal that the chain size monotonically decreases with increasing added salt concentration, indicating that the increases in specific viscosity cannot be assigned to chain expansion. Our results indicate that NaPSS, usually thought to be a model polyelectrolyte system, displays complex and unexpected rheological behavior when both the polymer and added salt concentration approach the molar range, where the Debye screening length becomes smaller than the Bjerrum length. The results support PG de Gennes' characterisation of polyelectrolytes as 'the least understood form of condensed matter'.
All data for this study are available here.
 Dilute polyelectrolyte solutions: recent progress and open questions (Soft Matter, 2024)
Dilute polyelectrolyte solutions: recent progress and open questions (Soft Matter, 2024)
Carlos G. Lopez, Atsushi Matsumoto and Amy Q. Shen
Polyelectrolytes are polymers bearing ionic groups along their backbone. In polar media, counterions dissociate, and the chains experience electrostatic repulsion between like-charged segments. Despite their importance in biological systems (DNA, RNA, or hyaluronan are polyelectrolytes) and their wide presence in formulated products, our understanding of polyelectrolytes remains relatively thin. This article presents a survey of the last 80 years of polyelectrolyte research and discusses current problems in polyelectrolyte physics.
In solutions of low ionic stregths, polyelectrolytes adopt highly stretched rod-like conformations. As salt is added electrostatic interactions are preogressivelys creened and their behaviour becomes more like that of neutral polymers. Scaling laws for the radius of gyration, diffusion coefficient, intrinsic viscosity and second virial coefficient with molar mass and added salt concentration are established and experimental data are compared with theoretical predictions. Scaling models can capture many of the observed experimental trends, but important disagreements are also observed. Key questions that are not well understood include 1) the rod-to-flexible transition that occurs when salt is added to a polyelectrolyte solution 2) the scaling of chain rigidity and excluded volume with solvent ionic strength 3) the transition between polyelectrolyte to ionomer regimes, and more broadly the role of dipolar forces in the solution behaviour of polyelectrolytes 4) how the fraction of condensed counterions depends on the solvent ionic strnegth and dielectric constant 5) whether underscreening occurs in concentrated electrolyte solutions.
 Controlled mechanical properties of poly(ionic liquid)-based hydrophobic ion gels by the introduction of alumina nanoparticles with different shapes (Soft Matter, 2024)
Controlled mechanical properties of poly(ionic liquid)-based hydrophobic ion gels by the introduction of alumina nanoparticles with different shapes (Soft Matter, 2024)
Yuna Mizutani, Takaichi Watanabe, Carlos G. Lopez and Tsutomu Ono
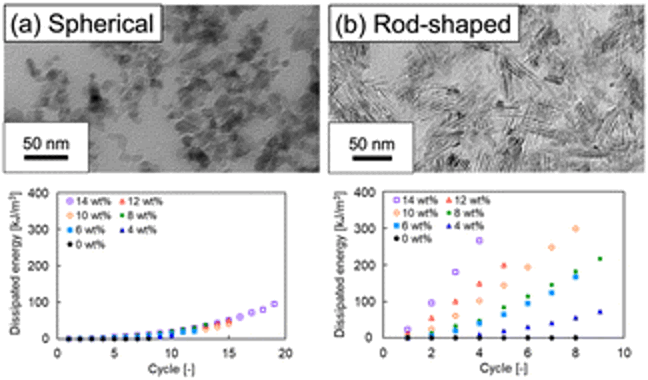
Ionic-liquid gels, or ion gels, are known for high ionic conductivity and CO2 absorption but face challenges in low mechanical strength. This study explores improving their practical applications by incorporating particles (silica, TiO2, and MOFs) into ion gels. Specifically, it investigates the impact of particle shape on mechanical properties using alumina/poly(ionic liquid) double-network ion gels with clustered alumina nanoparticles of different shapes. Results show enhanced mechanical strength in rod-shaped alumina/poly(ionic liquid) double-network ion gels compared to spherical ones. Cyclic tensile tests reveal energy dissipation through alumina network fracture, and TEM observation suggests that varying mechanical strength with particle shape is linked to differences in alumina particle aggregation structure, indicating the potential to tune ion gel strength by altering both particle type and shape.
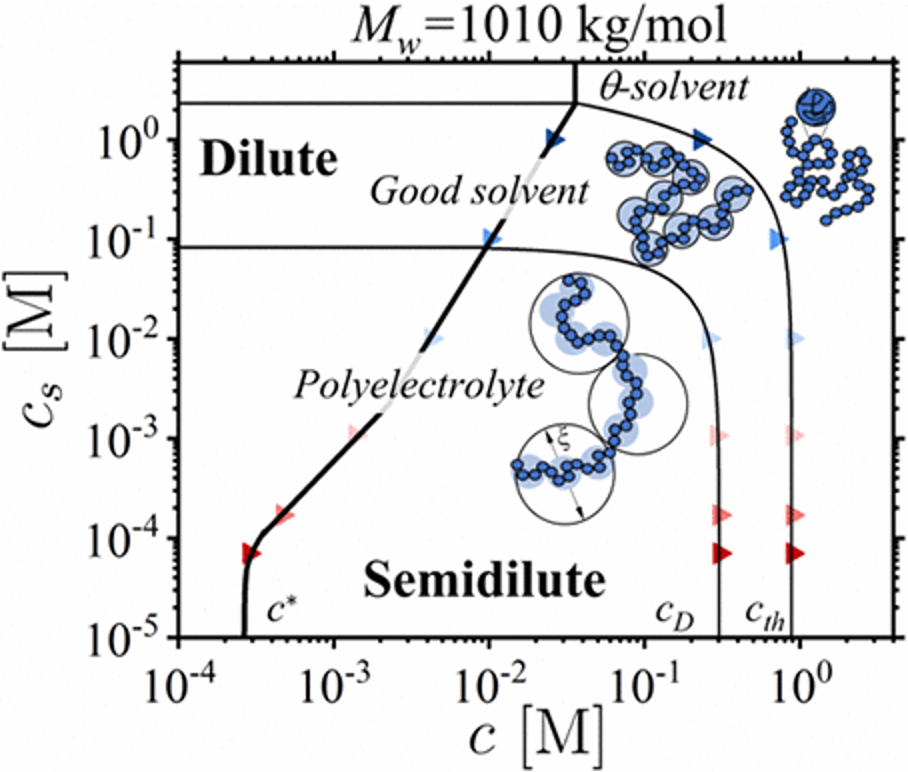 Salt Effect on the Viscosity of Semidilute Polyelectrolyte Solutions: Sodium Polystyrenesulfonate (Macromolecules, 2023)
Salt Effect on the Viscosity of Semidilute Polyelectrolyte Solutions: Sodium Polystyrenesulfonate (Macromolecules, 2023)
Anish Gulati, Michael Jacobs, Carlos G. Lopez, and Andrey V. Dobrynin
In this study, we investigated the viscosity of semi-dilute solutions of sodium polystyrenesulfonate in water, considering the amounts of polymer and salt present. To quantify viscosity, we used a scaling relationship based on the correlation length of the solution and the number of monomers in a given volume. The B-parameter, with specific values Bpe, Bg, and Bth, was determined based on factors like the fraction of charged monomers, the quality of the solvent, the length of polymer chains, and the interactions between monomers and the solvent.
These B-parameter values were obtained from the plateaus in the normalized specific viscosity as a function of monomer concentration. Extending this analysis to the entangled solution regime helped us determine the packing number of a chain of correlation blobs (P̃e), completing a set of parameters {Bpe, Bg, Bth, P̃e} that uniquely describe static and dynamic properties of polyelectrolyte solutions. This information was then used to create a diagram of states, calculate the fraction of free counterions, evaluate the energy of electrostatic blobs, and establish a concentration at which the solution transitions to the entangled regime.
 Electrostatically-Driven Collapse of Polyelectrolytes: The Role of the Solvent's Dielectric Constant
(Journal of Polymer Science, 2025)
Electrostatically-Driven Collapse of Polyelectrolytes: The Role of the Solvent's Dielectric Constant
(Journal of Polymer Science, 2025)